Sign up here to subscribe to the Grower2grower Ezine. Every two weeks you will receive new articles, specific to the protected cropping industry, informing you of industry news and events straight to your inbox.
Mar 2020
Why GACP & GMP

POSTPONED UNTIL FURTHER NOTICE:
Unfortunately due to current global circumstances and the new self-isolating restrictions for international travellers to prevent the spread of Covid-19, we have no option but to postpone the Grower2Grower Medical Cannabis workshop on the 31st of March.
Hopefully we will be able to reschedule later in the year subject to the control of the global pandemic.
I will make sure that those who registered for the postponed workshop, have first option to re-register, if and when we are able to proceed with the workshop in the near future.
Regards Stefan Vogrincic, MD Grower2Grower
Safety and efficacy during cultivation
In the upcoming Medical Cannabis workshop in New Zealand, Matt Hayes will discuss GACP and GMP. Below is a brief description of both GACP and GMP. In a recent article on MMJ Daily an Australian manufacture has gained valuable GACP certification in order to enable companies to comply with European regulations.
https://www.mmjdaily.com/article/9196653/australian-cannabis-poised-for-global-momentum/
WHY GACP & GMP? To ensure quality assurance, safety and efficacy during cultivation of plant materials used as sources of medicinal products (Active Pharmaceutical Ingredients, API).
To ensure manufacturing quality assurance, safety and efficacy, GMP quality must be built into each batch of product during all stages of the manufacturing process.
GACP-GMP guidelines provide a framework for compliance. You must validate your production processes.
Good Agricultural Collection Practices (GACP)
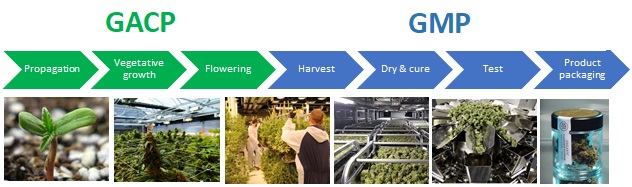
- Propagation>>>post-harvest.
- Produce a botanically-derived API.
- Validated & documented cultivation, germplasm & inputs, facility & climatic conditions, harvested product testing.
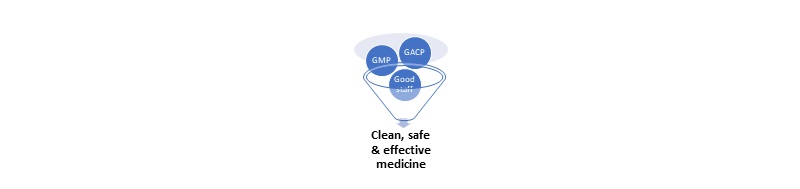
Good Manufacturing Practice (GMP)
- Post-harvest>>>packaging & labelling.
- Purification, processing and packaging of raw API into finished medicines
- Validated & documented processes, production inputs, facility & equipment, & finished product testing.
A product-focused approach helps to define processes, facilities, equipment and workflows: Product>Process> Licence>Site>Facility>Staff.
Each product has a defined specification, which defines production.
If you are interested in registering simply fill out the contact us form. Please complete the mandatory fields and type in the ‘Your Message’ box Register for MC Workshop, you will be sent a questionnaire with supporting detail to complete registration and payment. Once you have completed registration, for the workshop, exact location details will then be provided.
The cost to register is NZ $795 per person including GST. Numbers are strictly restricted.
Date of Workshop: Tuesday the 31st of March 2020
Time: 8.30 am to 4.00 pm
Location: Pukekohe, New Zealand
This will be an intensive one-day workshop running from 8.30 am to 4.00 pm. It is a unique opportunity to learn about High-Tech Cannabis production from one of the world’s leading experts in the cultivation of Medical Cannabis.
Morning tea and lunch will be provided.
This is a must attend workshop to start to understand the actual growing conditions/requirements for Medical Cannabis.

Major sponsor of the event is The NZ HORTICENTRE TRUST

The sponsorship from The New Zealand Horticentre Trust makes it possible to organise this educational event. We are very grateful for the generous support.
I appreciate your comments. Please feel free to comment on the grower2grower Facebook page:
https://www.facebook.com/StefanGrower2grower/
Article Written and compiled by Stefan Vogrincic, Consultant, Grower2Grower
Article Edited by Marie Vogrincic, Editor, Grower2Grower
CLASSIFIED
Subscribe to our E-Zine
More
From This Category

Tomato grower applies Tobre after contamination

KWS inaugurates new R&D facility in Uberlândia, Brazil

John van Santen joins the management of Metazet

Workshop for Auckland’s Korean tomato growers held last week

Could the Global Boom in Greenhouses Help Cool the Planet?

























